Abstract
The granulocyte colony stimulating factor receptor (CSF3R) is a critical regulator of neutrophil production with multiple alternatively spliced variants. The truncated CSF3R-V4 splice variant confers enhanced growth signals, and changes in its expression levels relative to the canonical V1 (wild type) isoform have been implicated in chemotherapy resistance and relapse of AML. We previously demonstrated that the CSF3R-V3 isoform, a variant of V1 with an insertion in the cytoplasmic domain, produces hypoproliferative signals in lymphoid cells in response to G-CSF. We also reported that expression of all three splice variants is significantly altered in AML, suggesting that aberrant CSF3R splicing is involved in the pathogenesis of some myeloid malignancies. The functional signaling capabilities of the different CSF3R isoforms in regulating granulopoiesis remain largely unknown. Herein, we describe a novel myeloid model system and show that the V3 and V4 isoforms generate opposing proliferative signals without effects on myeloid cell differentiation.
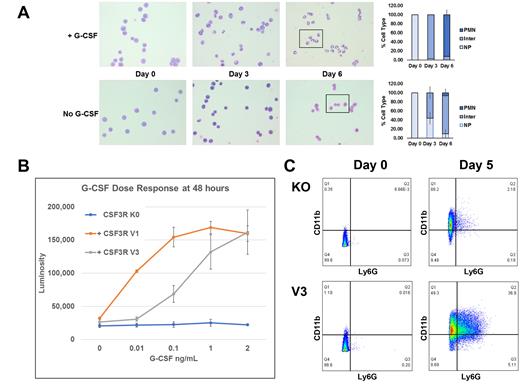
ER-HoxB8 cells are murine bone marrow progenitor cells ectopically expressing an ER-HoxB8 fusion protein, and in the presence of estradiol (E2) the fusion protein dimerizes producing a functional HoxB8 dimer which enforces self-renewal. Thus, in the presence of E2 these cells continually proliferate; however, when E2 is withdrawn they differentiate into mature granulocytes. Addition of G-CSF to culture medium of E2 ER-HoxB8 cells increases progenitor cell proliferation in a dose dependent manner (Figure 1A). Using CRISPR/Cas9, we knocked-out the endogenous murine Csf3r. As expected, ER-HoxB8-Csf3r-/- cells still produced mature neutrophils with E2 withdrawal and no increase in differentiation or proliferation of the knock-out cells (KO) was observed in response to G-CSF. The functional behavior of our ER-HoxB8-Csf3r-/- cells recapitulates the published phenotype of the Csf3r knock-out mouse, which exhibits severe neutropenia but has circulating neutrophils. ER-HoxB8 KO cells were transduced with human CSF3R splice variants and expression confirmed by immunoblot analysis using splice-variant specific antibodies. KO cells expressing the CSF3R-V3 demonstrated a hypoproliferative response to G-CSF with an ~40-fold increase in the EC50 relative to cells expressing CSF3R-V1 (Figure 1B), confirming our prior observations in the lymphoid BaF3 cell line. In contrast, KO cells expressing the truncated CSF3R-V4 variant hyperproliferated in response to G-CSF consistent with our previously published data in lymphoid cells.
Using multi-color flow cytometry with antibodies against CD117, CD11b, and Ly6G to identify progenitor, intermediately differentiated cells (NeuP), and mature neutrophils, we found that KO cells (like parental ER-HoxB8 cells) produced significant numbers of CD11b+/LyGG- NeuP cells upon E2 withdrawal and addition of G-CSF had no effect on differentiation. Transduction of ER-HoxB8 KO cells with the wild type human CSF3R-V1 restored their capacity to respond to G-CSF in a dose dependent manner. KO cells transduced with CSF3R-V3 displayed normal production of NeuP cells with E2 withdrawal, and addition of G-CSF produced a substantial increase in the numbers of mature neutrophils (CD11b+, Ly6G+) after 5 days in culture, relative to KO cells (Figure 2C). Thus, we have demonstrated that CSF3R-V3 is able to support the production of fully mature neutrophils. Notably, a G-CSF induced increase in the numbers of mature neutrophils was also evident in CSF3R-V4 transduced cells. Previous work by others indicated that CSF3R-V4 was not able to drive myeloid differentiation. We hypothesize that this difference in phenotype is due to a V4-dependent hyperproliferation of the neutrophil progenitors. On-going work is focused on the determination of the specific effects these CSF3R splice variants have on each stage of granulopoiesis.
In conclusion, using our novel engineered CSF3R model system, we confirm differential effects of CSF3R splice variants on myeloid cell proliferation and show sustained differentiation capacity of each isoform. Additional studies using this model system provide the opportunity for identification of new therapeutic targets for treatment of disorders of granulocyte production.
Avalos: JUNO: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal